📚 Learning Objectives:
✅ Understand how atoms behave differently in solids, liquids, and gases.
✅ Learn why solids are hard, liquids flow, and gases spread out.
✅ Relate the kinetic particle theory to real-world examples (ice, water, air).
📝 Quick Summary:
Solids, liquids, and gases behave differently because of how their atoms move and are arranged. Solids have tightly packed atoms that only vibrate, liquids have atoms that slide past each other, and gases have atoms that move freely and are far apart.
Everything in the world is made of tiny particles called atoms. These atoms are always moving, but how they move is different for solids, liquids, and gases. This is why a solid is hard, a liquid is wet, and a gas is… well, just air!
Think of it like a dance floor with a bunch of people on it:
Solids: A Very Crowded Dance Floor
In a solid (like a block of ice or a rock), the atoms are packed together so tightly they can’t move around. They’re like people in a very crowded room—all they can do is wiggle and vibrate in one spot.
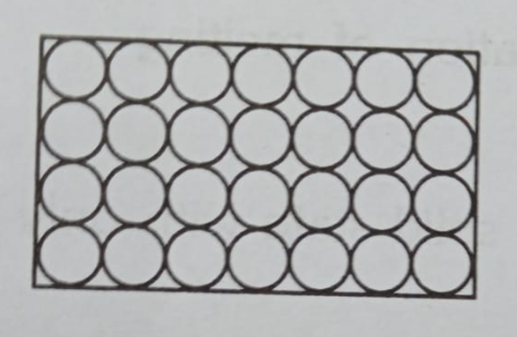
- Atoms are closely packed.
- They just vibrate in place.
- This is why solids are hard and hold their shape. You can’t walk through a wall because its atoms are stuck in place!
Liquids: A Bouncy Dance Floor
In a liquid (like water or juice), the atoms are a little further apart. They can slide and bump past each other, but they still stick together.
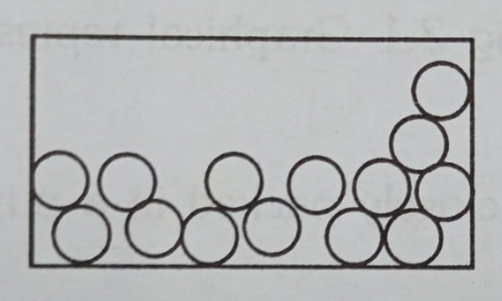
- Atoms are close but can slide past each other.
- This is why liquids can flow and take the shape of a container. It’s like people on a dance floor who can move around and touch shoulders, but still stay in the same area.
- You can walk through water, but it’s much harder than walking through air because the atoms are closer together.
Gases: An Empty Dance Floor
In a gas (like the air we breathe or the helium in a balloon), the atoms are really far apart from each other. They fly around freely, bouncing off the walls and other atoms.
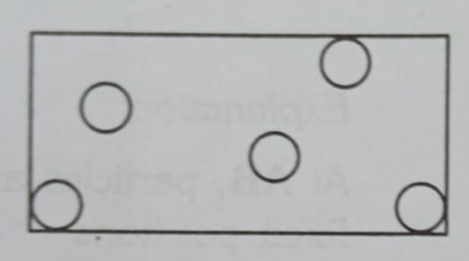
- Atoms are far apart.
- Most of the space is empty.
- This is why you can easily walk through the air. The gas atoms are so spread out that they don’t get in your way!
So, the next time you see something—whether it’s a solid, a liquid, or a gas—remember that the only difference between them is how their tiny atoms are arranged and how they are moving!
Master Chemistry concepts the fun way — start your learning journey today!
🌐 Website:www.conceptacademia.org
📞 Phone: +92-300-4305408
📧 Email:info@conceptacademia.org