📚 Learning Objectives:
✅ Discover how the arrangement of atoms determines the properties of materials.
✅ Learn why graphite is soft and conducts electricity, while diamond is hard and does not conduct electricity — even though both diamond and graphite are carbon.
✅ Understand electronic configuration and how it predicts chemical behavior.
📝 Quick Summary:
The structure of atoms — how they bond and how their electrons are arranged — determines everything about a material’s properties. From the hardness of diamonds to the slipperiness of graphite, chemistry is all about structure!
Have you ever wondered why a shiny diamond is so hard, but the graphite in your pencil is so soft? Or why some elements are metals, while others are gases? The answer lies in something you can’t even see: atoms and how they’re built.
The Magic of Structure
Why diamond and graphite are so different, even though they’re both made of only carbon atoms.
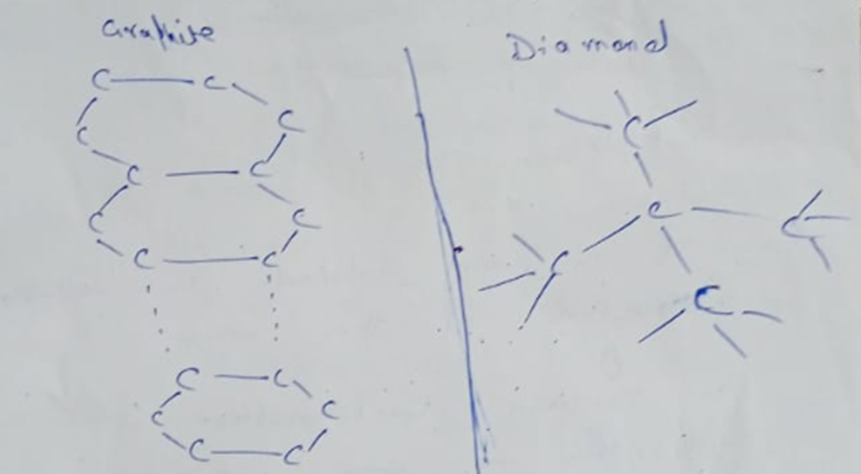
- Graphite: In graphite, carbon atoms are arranged in flat sheets that are stacked on top of each other. The bonds between the sheets are very weak, so they can easily slide apart. This makes graphite soft and slippery, which is why it’s used in pencils and as a lubricant. Because of its unique structure, it’s also the only non-metal that can conduct electricity!
- Diamond: In diamond, carbon atoms are locked together in a super-strong, 3D structure. Every atom is tightly bonded to four others, creating a rigid lattice. This is what makes diamond the hardest material on Earth! Because its electrons are all held in these strong bonds, it can’t conduct electricity.
As you can see, the way atoms are arranged directly determines the material’s properties.
The Building Blocks of Chemistry
To really understand why things act the way they do, we have to look inside the atom itself. This is where we learn about protons, neutrons, and electrons.
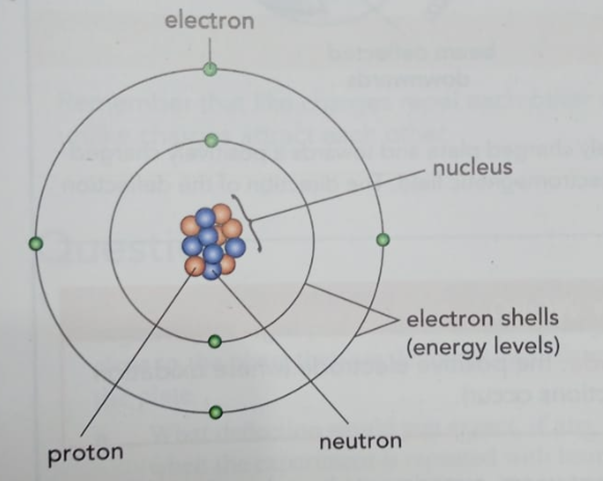
- The Nucleus: At the very center of an atom is the nucleus, which holds the protons and neutrons. Think of it as the atom’s core.
- Electrons: The electrons are tiny, fast-moving particles that orbit the nucleus in shells, like planets around the sun.
The most important part of this is that electrons can jump from one atom to another. This movement is what causes all the amazing changes we see in chemistry!
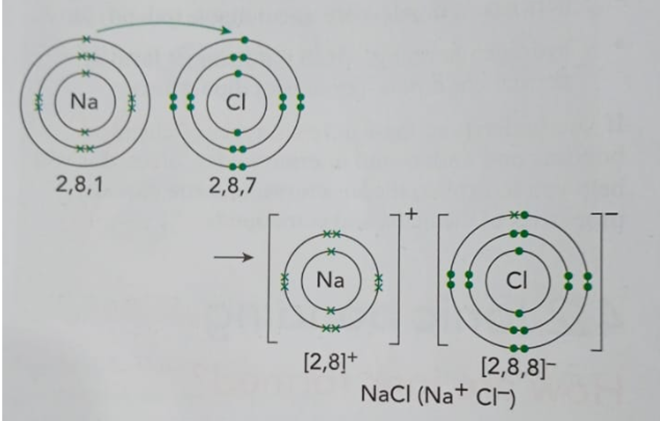
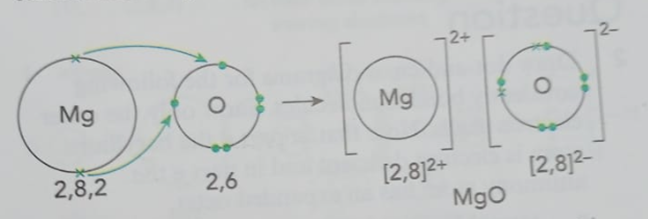
To understand these changes, we need to know about electronic configuration. This is just a fancy name for how electrons are arranged in their shells around the nucleus.
- The number of electrons in an atom’s outermost shell tells us a lot about what it will do. This is why sodium is a metal, silicon is a metalloid, and chlorine is a non-metal.
By understanding how electrons are arranged, we can predict an element’s behavior and what kinds of reactions it will have. Without knowing an atom’s structure and electronic configuration, we’d be completely lost!
This is why learning about atoms and their structure is the first and most important step on your journey to understanding chemistry. Once you master this, you’ll be ready to learn about how atoms combine to form molecules—the next big milestone!
Unlock the secrets of chemistry — start learning with Concept Academia today!
🌐 Website:www.conceptacademia.org
📞 Phone: +92-300-4305408
📧 Email:info@conceptacademia.org